BOTOX® Cosmetic & Other Neurotoxins
- What Are Neurotoxins?
- Benefits of BOTOX® Cosmetic and Other Neurotoxins
- Neurotoxin Options
- BOTOX® Cosmetic and Other Neurotoxins Treatment
- BOTOX® Cosmetic and Other Neurotoxins Safety
- BOTOX® Cosmetic and Other Neurotoxins Side Effects
- BOTOX® Cosmetic and Other Neurotoxins Longevity
- BOTOX® Cosmetic and Other Neurotoxins Cost
- BOTOX® Cosmetic and Other Neurotoxins Alternatives
What Are BOTOX® Cosmetic and Other Neurotoxins?
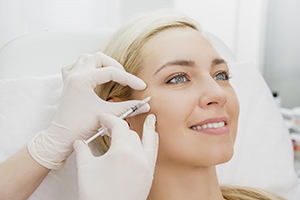 BOTOX® Cosmetic, Dysport®, and XEOMIN® are botulinum toxin type A (BTXA) injectables that are processed and purified to produce a sterile therapeutic neuromuscular blocking agent suitable for injection. When administered properly, they can temporarily relax the treated muscles to inhibit the release of acetylcholine. As a result, these injections can effectively treat lines and wrinkles caused by muscle contraction during facial expressions, such as frowning or squinting.
BOTOX® Cosmetic, Dysport®, and XEOMIN® are botulinum toxin type A (BTXA) injectables that are processed and purified to produce a sterile therapeutic neuromuscular blocking agent suitable for injection. When administered properly, they can temporarily relax the treated muscles to inhibit the release of acetylcholine. As a result, these injections can effectively treat lines and wrinkles caused by muscle contraction during facial expressions, such as frowning or squinting.
 Board-certified plastic surgeon Joseph DiBello, MD, FACS is highly experienced in performing BOTOX® Cosmetic, Dysport®, and XEOMIN® injections to address both cosmetic and medical-related concerns. If you are interested in treatment in the Philadelphia area, we encourage you to continue reading the information below to learn more about these BTXA injectables, or simply contact our practice today to schedule a consultation with Dr. DiBello.
Board-certified plastic surgeon Joseph DiBello, MD, FACS is highly experienced in performing BOTOX® Cosmetic, Dysport®, and XEOMIN® injections to address both cosmetic and medical-related concerns. If you are interested in treatment in the Philadelphia area, we encourage you to continue reading the information below to learn more about these BTXA injectables, or simply contact our practice today to schedule a consultation with Dr. DiBello.
What Are the Benefits of BOTOX® Cosmetic & Neurotoxins?
Key benefits of treatment with BOTOX® Cosmetic and other neurotoxins often include:
- A smoother and more youthful-looking facial aesthetic
- Significant reduction of bunny lines, 11 lines, worry creases, forehead lines, crow’s feet, and additional concerns
- Generally a very safe and quick procedure
- Usually no downtime needed; patients can typically resume normal activities right away, although exercise/exertion should be avoided for approximately four to six hours after treatment
In addition, neurotoxins have been approved to treat certain medical conditions, such as:
- Crossed eyes (strabismus)
- Eyelid spasms (blepharospasm)
- Cervical dystonia (spastic muscle disorder with the neck)
- Spasticity disorders in children with cerebral palsy
- Motor disorders of the facial nerve (VII cranial nerve)
- Migraine headaches
- Colorectal disorders
- Excessive perspiration (hyperhidrosis) disorders of the armpit and hands
- Musculoskeletal pain disorders
Which Neurotoxin Injectable Should I Choose?
Dr. DiBello offers the following popular neurotoxin injectables:
BOTOX® Cosmetic
BOTOX® Cosmetic (cosmetic form of onabotulinumtoxinA, developed by Allergan) has FDA approval for temporary improvement in the appearance of the following aesthetic concerns for adults:
- Moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity (20 units, approved in 2000)
- Moderate to severe lateral canthal lines (“crow’s feet”) associated with orbicularis oculi activity (24 units—12 per side—approved in 2013)
- Moderate to severe forehead lines associated with frontalis muscle activity (20 units, approved in 2017).*
Dysport®
Dysport® (abobotulinumtoxinA, created by Galderma) was given FDA approval in 2009 for the treatment of glabellar lines (50 units). While very similar in composition to BOTOX® Cosmetic, Dysport® contains fewer proteins, which can allow it to spread further. * Additionally, Dr. DiBello has found the gradual onset of effects for BOTOX® Cosmetic and XEOMIN®—which is about three to five days—to differ slightly from Dysport®, which usually becomes noticeable within one to three days (and at a different dosage—2.5 units of Dysport® to each unit of BOTOX® Cosmetic).
XEOMIN®
XEOMIN® (incobotulinumtoxinA, produced by Merz) was FDA approved in 2010, also for the treatment of glabellar lines (20 units). On a molecular level, a key difference between these three BTXA injectables is that XEOMIN® has one less protein side chain than Dysport® and BOTOX® Cosmetic, making it potentially effective for patients who have developed tolerance or immunity to Dysport® or BOTOX® Cosmetic—but with a slightly shorter duration of effectiveness.
While BOTOX® Cosmetic is the only BTXA injectable with FDA approval for all three treatment areas (forehead, glabella, and lateral canthi), both Dysport® and XEOMIN® are routinely used for these areas as well. Treatment of areas such as the perioral area (around the mouth) and neck bands, while commonly performed, are not specifically approved by the FDA, and are therefore considered “off label.”
What Is Treatment with BOTOX® Cosmetic & Other Neurotoxins Like?
Dr. DiBello personally performs all of BOTOX® Cosmetic and BTXA injections at DiBello Plastic Surgery. You will consult directly with him and receive an individualized plan tailored to your needs, goals, and budget.
During treatment, Dr. DiBello will carefully administer the selected BTXA product directly into the muscles in the chosen area(s) in a series of injections with extremely small needles. Due to his gentle approach and seasoned technique, most patients experience little to no discomfort (rarely anything more than a slight “pinching” sensation). For added comfort, cool compresses will be applied at the conclusion of treatment.
The entire procedure takes approximately 10 to 15 minutes to perform, and the expression lines in the treatment area(s) will usually appear noticeably softer within a few days.
After this non-surgical, minimally invasive treatment, you will likely be able to resume normal daily routines as soon as you leave our office. That said, in order to help ensure an optimal outcome, you will need to avoid exercise and any strenuous activities for at least four to six hours.
To see this procedure animated, click here.
Are Neurotoxin Injectables like BOTOX® Cosmetic Safe?
The Aesthetic Society® maintains that BTXA injections for aesthetic purposes appear to be safe and effective.* Patients who show early signs of aging, as well as those who may not be suitable candidates for more extensive aesthetic facial surgery, may be good candidates for this procedure.
Furthermore, certain medications (some antibiotics, anti-inflammatories, or aspirin) and even some vitamins and herbs may increase the potency of BTXA and could increase bleeding and bruising at the time of injection. Therefore, you should be candid with Dr. DiBello about all medications and supplements. Pregnant or nursing women should postpone undergoing these procedures. It is not known whether injection of BTXA has any effect on a fetus or whether it is found in breast milk. Dr. DiBello advises all patients who receive the COVID-19 vaccine/booster to avoid BOTOX® Cosmetic and BTXA injections within 2 weeks of the vaccine shot (two (2) weeks before or two (2) weeks after).
Are There Side Effects of BOTOX® Cosmetic & BTXA Injections?
Potential side effects of BOTOX® Cosmetic, Dysport®, and XEOMIN® include local numbness, swelling, bruising, or a burning sensation during injection. Some patients have also reported temporary headaches and nausea. Since flu-like symptoms are a rare, but possible, side effect of BOTOX® injections, you should avoid receiving a flu shot (either with the COVID-19 booster, or separately) within two weeks of treatment.
Very rarely, the BTXA solution can spread beyond the treatment area, which can cause botulism-like signs and symptoms such as breathing problems, trouble swallowing, muscle weakness, and slurred speech. With this in mind, most complications are of short duration and can be avoided with proper injection techniques. A small percentage of patients are reported to experience no improvement at all.
Ultimately, it is important to note that although the side effects mentioned above are possible, the probability of their occurrence following BOTOX® Cosmetic, Dysport®, and XEOMIN® is greatly minimized when treatment is performed by a board-certified plastic surgeon who is both skilled and experienced with these injectables, such as Dr. DiBello.
How Long Do BOTOX® Cosmetic, Dysport®, & XEOMIN® Results Last?
While most patients are very pleased with the results of treatment, the outcome is not permanent. BOTOX® Cosmetic, Dysport®, and XEOMIN® each typically last for approximately three to four months for most patients, though the duration of XEOMIN® may be slightly less for some individuals.
Generally, optimal results for smoothing the wrinkles/lines can be seen after cumulative (repeated) injections, followed by maintenance. A minimum period of three months should be waited between injections of BTXA to avoid the development of immunity to the product. Dr. DiBello will personally advise you as to the recommended treatment, as well as maintenance program, that he individualizes for you.
How Much Do BOTOX® Cosmetic & Other Neurotoxins Cost?
At our Philadelphia practice, the cost of BOTOX® Cosmetic, Dysport®, and XEOMIN® treatments usually ranges between $400.00 and $600.00. Of course, exact pricing will be dependent upon the specific BTXA injectable you receive, how many areas/concerns are targeted, and the number of units administered. At the time of your consultation, Dr. DiBello can provide a personalized quote after performing a thorough evaluation and developing a custom treatment plan based on your unique needs and goals.
Are There Alternatives to BOTOX® Cosmetic & BTXA Injections?
Dr. DiBello offers multiple alternatives to BOTOX®, Dysport®, and XEOMIN® for patients who are interested in other facial rejuvenation options, or for those whose aesthetic concerns would be more effectively addressed with a different treatment option.
Dermal fillers—including the JUVÉDERM® and Restylane® product families—can be an excellent option for many individuals seeking to reduce moderate to significant lines and wrinkles found around the mouth and throughout the mid to lower face. Additionally, microdermabrasion and chemical peels have the ability to diminish fine lines while smoothing the skin. In some cases, a brow lift and/or facelift surgery may be best suited for comprehensive, long-lasting enhancement.
During your consultation, Dr. DiBello will cover all options available to you, helping you decide which treatment is ideal for your needs, goals, and expectations.
If you have additional questions about BOTOX® Cosmetic and/or other BTXA injections, or if you wish to book an appointment with Dr. DiBello, please don’t hesitate to contact our office today.
